Fertilizer in Waterways Can Lead to an Increase in _______.
Abstract
Fertilizers are added to crops in gild to produce enough nutrient to feed the human population. Fertilizers provide crops with nutrients like potassium, phosphorus, and nitrogen, which allow crops to abound bigger, faster, and to produce more food. Nitrogen in detail is an essential nutrient for the growth of every organism on Globe. Nitrogen is all around us and makes up about 78% of the air you breathe. However, plants and animals cannot use the nitrogen gas in the air. To abound, plants require nitrogen compounds from the soil, which can be produced naturally or be provided past fertilizers. However, applying excessive amounts of fertilizer leads to the release of harmful greenhouse gases into the atmosphere and the eutrophication of our waterways. Scientists are currently trying to find solutions to reduce the environmentally harmful effects of fertilizers, without reducing the amount of nutrient we tin produce when using them.
What Is Fertilizer?
Fertilizer is any substance or textile added to soil that promotes found growth. In that location are many fertilizer varieties, and well-nigh contain nitrogen (North), phosphorus (P), and potassium (K). In fact, fertilizers sold in stores accept an Due north-P-K ratio on their packaging. Fertilizers are practical all around the earth to go along lawns green and to produce more crops in agricultural fields. Fertilizers can exist divided into three groups:
- Mineral fertilizers (phosphorus and potash) are mined from the environment and crushed or chemically treated before being applied.
- Organic fertilizers (manure and compost) are made from animal feces, and plant or animal decomposed thing.
- Industrial fertilizers (ammonium phosphate, urea, ammonium nitrate) are produced industrially by humans through chemic reactions.
While organic and mineral fertilizers take been used to increase ingather yields in agriculture for a long fourth dimension, industrial fertilizers are a relatively new evolution. Even and then, industrial fertilizers are the most widely used fertilizers today.
Why Practise We Need Nitrogen-Containing Fertilizers?
Nitrogen is one of the elements, or nutrients, that all living things (microorganisms, plants, and animals) need to grow. Although, there is a lot of nitrogen all effectually united states (~78% of the air we breathe), most of the nitrogen on World is present as a colorless and odorless gas, called nitrogen gas (Northward2). Unfortunately, plants and animals cannot straight utilise nitrogen gas. As humans, we go our nitrogen from the food nosotros eat. High poly peptide foods similar meat, fish, nuts, or beans are loftier in nitrogen. Plants get their nitrogen from the soil and nitrogen is the most common nutrient to limit plant growth. There are two ways nitrogen gas is naturally transformed or "fixed" into nitrogen-containing compounds that can end up in soil, without human being intervention (Effigy 1):
- Lightning: Lightning strikes generate enough energy to split up nitrogen gas in the atmosphere creating nitrogen-containing compounds, which finish upwardly in soil.
- Biological nitrogen fixation: Some microorganisms can use nitrogen gas directly equally a nutrient. These specialized microorganisms convert nitrogen gas to ammonium (NH4 +) and are called "nitrogen fixers." Some nitrogen-fixing microorganisms live in soil, and some can course a close human relationship with the roots of certain plants, similar beans or clover.
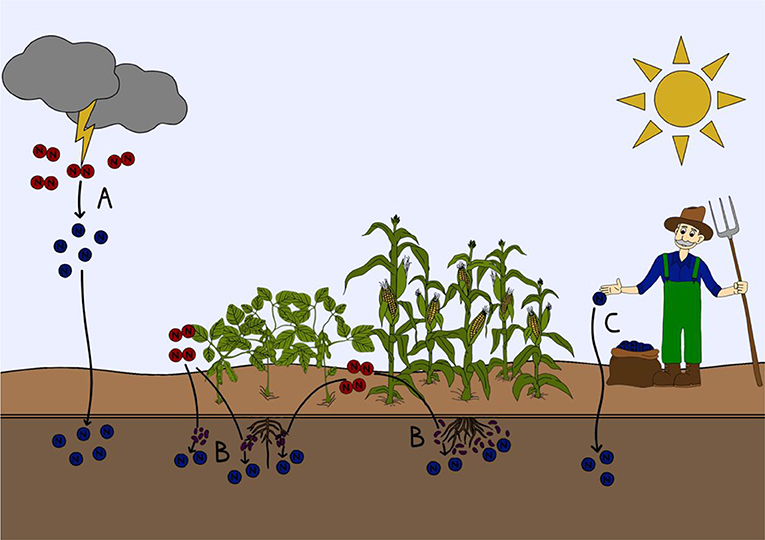
- Effigy 1 - How nitrogen gas fixed into a form that can be used by plants and animals.
- (A) Lightning strikes tin can split nitrogen gas (blood-red) in the temper. The newly created nitrogen compounds (blue) so fall down onto soils and naturally fertilize them. (B) Specialized nitrogen fixing microorganisms in the soil or attached to plant roots can transform nitrogen gas into nitrogen compounds that can be used by plants and animals. (C) Nitrogen gas can be transformed into usable nitrogen compounds industrially with the Haber-Bosch process to make fertilizers, which can be directly applied to soils.
Notwithstanding, even with all this natural nitrogen fixation , low nitrogen levels in soils often still limit found growth. This is why most fertilizers contain nitrogen compounds and why industrial fertilizers are essential in social club to produce enough crops to feed the human population. Humans at present add as much or more than industrially fixed nitrogen (~150 billion kilograms) to the surround each twelvemonth, than is naturally fixed [i, ii]. One hundred and 50 billion kilograms (~330 billion pounds) of annihilation is hard to imagine, but this is equal to the weight of ~24 million fully grown developed elephants!
How Are Nitrogen-Containing Industrial Fertilizers Produced?
As mentioned, most nitrogen on Earth is present as nitrogen gas, which is unusable for plants and animals. In the early 1900'southward, scientists discovered how to transform nitrogen gas from the atmosphere into nitrogen-containing compounds that could be used to fertilize soils (Figure ane). This industrial fixation is called the Haber-Bosch process . Almost all the nitrogen in industrial fertilizers is fixed through the Haber-Bosch procedure.
This industrial fixation of nitrogen is performed in chemical laboratories and large factories all over the world. The Haber-Bosch process requires that nitrogen gas be mixed with hydrogen gas (H2) and put under enormous pressure (200 times atmospheric pressure). This is the pressure you would feel if you lot pigeon 2,000 meters (~half-dozen,500 anxiety) underneath the sea, which is a longer distance than six Eiffel Towers stacked on elevation of one another! This pressurized gas mixture is then heated to very high temperatures (450°C/842°F). Sustaining these high pressures and temperatures requires a huge amount of energy. The Haber-Bosch process is estimated to consume 1–2% of the world'southward energy supply each year [two].
Why Do Nosotros Use So Much Nitrogen-Containing Industrial Fertilizer?
The short respond is that nitrogen-containing fertilizers help crop plants grow faster and helps to produce more than crops. This allows agricultural land to be used more efficiently considering fertilized state produces more than nutrient. In fact, the invention of industrial fertilizers is 1 of the main reasons the Earth's population has grown so quickly in the last lx–seventy years. Before the widespread apply of industrial fertilizers in the 1960's, information technology took ~123 years for the Earth'south population to double from 1 to 2 billion (1804–1927). However, it merely took ~45 years (1974–2019) for the Earth's population to double from 4 to 8 billion. Now, we are so dependent on nitrogen fertilization that we would merely be able to produce enough food to feed ~fifty% of the world'southward population without information technology [1, 2].
Where Does the Nitrogen From Nitrogen-Containing Fertilizer Finish Up?
The crops take it up of class! Unfortunately, that is not the end of the story. For a more detailed wait at all the reactions in the nitrogen bicycle, you should read this Young Minds Article: "What is the Nitrogen Cycle and Why is it Key to Life" [3]. In an average agronomical field, only ~50% of the nitrogen from fertilizers is used by crops [4]. Then, while fertilizers make crops abound better and faster, half of the fixed nitrogen nosotros add together is lost. Imagine that—we lose the equivalent of 12 meg nitrogen elephants (~165 billion pounds) every year! The lost nitrogen can cease upwards in the temper or it can be done out of the soil and end up in waterways, such as groundwater, streams, lakes, rivers, and oceans (Figure 2). This lost nitrogen causes a variety of environmental problems [2].
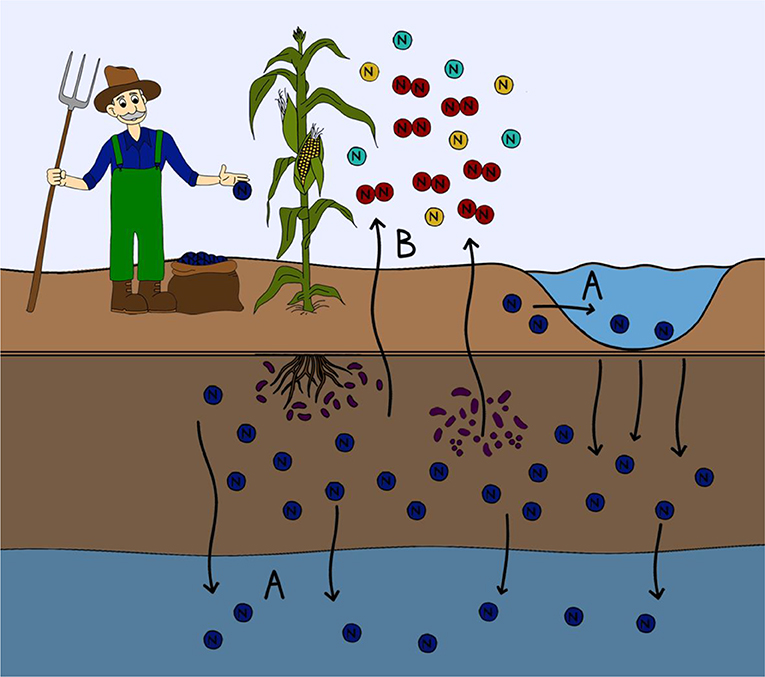
- Figure 2 - Where nitrogen ends up in the environment.
- Nitrogen from fertilizers that is not taken upwardly by plants can be lost from the soil. (A) Nitrogen can be leached from the soil and enter into waterways either to a higher place basis (lakes, streams, rivers, or oceans) or into ground water. Nitrogen leaching into aquatic ecosystems tin can lead to harmful algal blooms and the eutrophication of waterways. (B) Some microorganisms are able to transform the nitrogen in fertilizer into a variety of unlike nitrogen containing gases. This gaseous nitrogen tin can then be lost to the temper in the form of greenhouses gases.
What Ecology Problems Do Nitrogen-Containing Fertilizers Cause?
Some soil microorganisms can transform nitrogen provided in fertilizers into nitrogen-containing gases, which get released into the atmosphere like the greenhouse gas nitrous oxide (North2O). Greenhouse gases are one of the main factors accelerating global warming. Nitrous oxide has a warming potential ~300 times greater than the most ordinarily mentioned greenhouse gas, carbon dioxide (CO2).
In waterways, the addition of external nutrients (like backlog nitrogen) is called eutrophication . Eutrophication is an unwanted fertilization of a waterway and it promotes the growth of microorganisms, algae, and plants, just like the fertilization of soil. All the same, the fast growth of microorganisms and plants can utilize upwardly all the oxygen in these waterways and turn them into and so-called dead zones, because aquatic animals cannot alive without oxygen. Eutrophication can also pb to the growth of algal species that produce toxic chemicals, called harmful algal blooms .
While nosotros need nitrogen from fertilizers in our agricultural soils, we exercise not need or want additional nitrogen in our atmosphere or waterways. This means we have to residue the positive benefits of nitrogen fertilization (more than food) with the negative consequences of backlog fertilizer (environmental problems) [1, 2]. Scientists are currently working to discover this residue to improve our current state of affairs.
What Fertilizer Related Research Is Currently Being Done?
1 main goal of fertilizer related inquiry is to decrease the amount of industrially fixed nitrogen that is lost (~12 1000000 elephants worth) to the atmosphere and waterways. This solution is called improving the nitrogen use efficiency of agronomical environments. Here are a few examples of ongoing fertilizer inquiry:
Microbiologists and soil scientists are working on means to improve field conditions to promote the growth of naturally occurring soil nitrogen-fixing bacteria. In addition, they are also working on ways to forestall the growth of soil microorganisms that contribute to stock-still nitrogen being lost to the atmosphere or waterways (Figure 3). Together, this would reduce the overall amount of nitrogen-containing fertilizer needed to go the aforementioned crop yield.
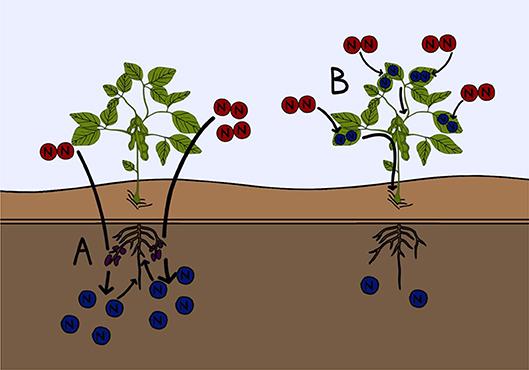
- Effigy three - Two examples of current inquiry into improving fertilizer efficiency.
- (A) Microbiologists and soil scientists are working to meliorate the growth of nitrogen fixing microorganisms constitute in the soil, to increment biological nitrogen fixation. This will increase the nitrogen content of the soil (blue). (B) Constitute biologists are working to create crop plants that are capable of fixing nitrogen gas (ruby) directly from the temper into their tissues. This would reduce the need to add nitrogen containing fertilizers to these crops.
Chemists are working on designing fertilizers that are stable in soils over longer time periods and are less probable to be broken downwardly by microorganisms. These wearisome release fertilizers release little bits of nutrients at a fourth dimension, and so nutrients are available throughout the lifetime of the crops. This approach is still dependent on nitrogen-containing fertilizers, merely it would reduce the amount of fertilizer needed and decrease the nitrogen lost.
Plant biologists are trying to genetically engineer crops that would require less nitrogen from fertilizers [5]. These crops would be able to prepare their own nitrogen from nitrogen gas, just like the specialized nitrogen-fixing microorganisms. These crops would demand less fertilizer to produce the aforementioned ingather yield (Figure 3).
Calculator scientists and soil scientists are working together to design smart fertilization systems, which tin can monitor soil and air atmospheric condition in agricultural fields. These systems can then add together small amounts of fertilizer only when needed. This minimizes the amount of fertilizer added, makes fertilizer additions targeted to the crops needs, and decreases the corporeality of nitrogen lost.
Summary
Fertilizers provide crops with essential nutrients like nitrogen, and so that the crops grow bigger, faster, and produce more than food. However, applying too much fertilizer tin exist a problem because it leads to the release of greenhouse gases and eutrophication. Scientists are currently trying to find solutions to reduce the amount of fertilizers needed, without reducing the corporeality of nutrient produced.
Glossary
Nitrogen Fixation: ↑ The process of converting nitrogen gas into nitrogen containing compounds. Nitrogen fixation tin can occur naturally through lightning strikes, be performed by specialized microorganisms, or exist accomplished industrially.
Haber-Bosch Procedure: ↑ An industrial nitrogen fixation process that can be performed in a laboratory to produce fertilizer components. It was discovered by and is named for the scientists Fritz Haber and Carl Bosch.
Greenhouse Gases: ↑ Gases that trap heat in the atmosphere much like the roof of a greenhouse traps heat to protect the plants growing in it from cold conditions and frost.
Eutrophication: ↑ A change in an surround's nutrient status caused past high levels of nutrients (nitrogen or phosphorus) inbound waterways (lakes, rivers, or oceans). One major result is harmful algal blooms and the loss of aquatic life.
Harmful Algal Blooms: ↑ When blue-green alga and algae abound very fast because of large amounts of nutrients (nitrogen or phosphorus) nowadays in the waters they alive in. These cyanobacteria and algae release harmful chemicals—toxins—into the waterway.
Conflict of Interest
The authors declare that the research was conducted in the absenteeism of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Linnea Kop graciously created and granted permission for the utilize of her illustrations for all of the figures used in this commodity.
References
[i] ↑ Galloway, J. N., Leach, A. Grand., Erisman, J. West., and Bleeker, A. 2017. Nitrogen: the historical progression from ignorance to knowledge, with a view to future solutions. Soil Res. 55:417–24. doi: 10.1071/SR16334
[2] ↑ Erisman, J. W., Galloway, J. N., Dice, N. B., Sutton, Chiliad. A., Bleeker, A., Grizzetti, B., et al. 2015. Nitrogen: Besides Much of a Vital Resource. Science Cursory. Zeist: WWF Netherlands.
[3] ↑ Aczel, M. 2019. What is the nitrogen cycle and why is it cardinal to life? Forepart. Young Minds 7:41. doi: ten.3389/frym.2019.00041
[4] ↑ Hirel, B., Tétu, T., Lea, P. J., and Dubois, F. 2011. Improving nitrogen employ efficiency in crops for sustainable agronomics. Sustainability iii:1452–85. doi: ten.3390/su3091452
[5] ↑ Good, A., 2018. Toward nitrogen-fixing plants: a concerted research try could yield engineered plants that can directly set nitrogen. Science 359:869–seventy. doi: 10.1126/science.aas8737
Source: https://www.frontiersin.org/articles/486326
0 Response to "Fertilizer in Waterways Can Lead to an Increase in _______."
Enviar um comentário